What is CCR5 Delta32?
|
|
|
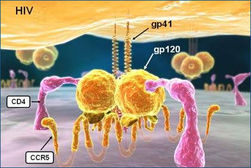
"CCR5-delta32" is a deletion mutation of a gene which only 1% of the total population has two copies of this gene and individuals who carry two copies of this genetic mutation are immune to Smallpox, The Bubonic Plague (Black Death) and resistant to HIV, the virus that causes AIDS. Up to 20% of the population carry only one copy of this genetic mutation depending on your background and although they still run a significant risk of contracting HIV, the progress of the disease is greatly reduced and can result in a longer life expectancy.
What is CCR5-delta32?
Cysteine-cysteine chemokine receptor 5 (CCR5) is found in the cell membranes of many types of mammalian cells, including nerve cells and white blood cells. The role of CCR5 is to allow entry of chemokines into the cell—chemokines are involved in signaling the body’s inflammation response to injuries. The gene that codes for CCR5 is situated on human chromosome 3. Various mutations of the CCR5 gene are known that result in damage to the expressed receptor. One of the mutant forms of the gene is CCR5-delta32, which results from deletion of a particular sequence of 32 base-pairs. This mutant form of the gene results in a receptor so damaged that it no longer functions. But surprisingly, this does not appear to be harmful.
Why CCR5-delta32 is Resistant to HIV (Please Watch The Video Above)
This mutation can be advantageous to those individuals who carry it. The virus HIV normally enters a cell via its CCR5 receptors, especially in the initial stage of a person becoming infected. But in people with receptors crippled by the CCR5-delta32 mutation, entry of HIV by this means is blocked, providing resistance to HIV for people with delta 32 mutation on both genes (called homozygous carriers) and greatly slowing progress of the disease in people with a delta 32 mutation on one of the two genes (called heterozygous carriers).
What is CCR5-delta32?
Cysteine-cysteine chemokine receptor 5 (CCR5) is found in the cell membranes of many types of mammalian cells, including nerve cells and white blood cells. The role of CCR5 is to allow entry of chemokines into the cell—chemokines are involved in signaling the body’s inflammation response to injuries. The gene that codes for CCR5 is situated on human chromosome 3. Various mutations of the CCR5 gene are known that result in damage to the expressed receptor. One of the mutant forms of the gene is CCR5-delta32, which results from deletion of a particular sequence of 32 base-pairs. This mutant form of the gene results in a receptor so damaged that it no longer functions. But surprisingly, this does not appear to be harmful.
Why CCR5-delta32 is Resistant to HIV (Please Watch The Video Above)
This mutation can be advantageous to those individuals who carry it. The virus HIV normally enters a cell via its CCR5 receptors, especially in the initial stage of a person becoming infected. But in people with receptors crippled by the CCR5-delta32 mutation, entry of HIV by this means is blocked, providing resistance to HIV for people with delta 32 mutation on both genes (called homozygous carriers) and greatly slowing progress of the disease in people with a delta 32 mutation on one of the two genes (called heterozygous carriers).
Current world-wide frequency distribution of CCR5-Δ32 allele frequencies.
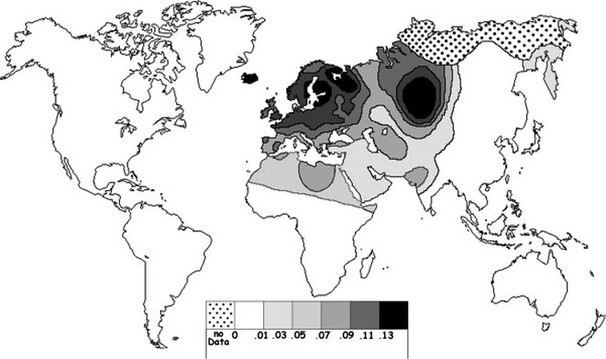
The delta 32 mutation is more prevalent in some racial backgrounds than in others. Population studies of the Caucasian population of western European ancestry revealed that approximately 1% of people were homozygous (2 copies) for the delta 32 mutation and up to 20% of people were heterozygous (1 copy). Within the United States, Canada and Australia, the frequency is 8% to 10% within the Caucasian background individuals, but less than 1% in the Afro-American populations. There is also a very low frequency of the mutation among the population in eastern Asia but much higher towards western Asia.
History of CCR5-Delta 32
For a disease-causing microorganism to infect the human body there must be a gateway or portal through which it enters into human cells. The plague bacterium works this way, hijacking the white blood cells sent to eliminate it. Traveling inside the white blood cells to the lymph nodes, the bacteria break out and attack the focal point of the human immune system. Dr. Stephen O'Brien felt that the mutated CCR5 gene, delta 32, may have prevented the plague from being able to enter its host's white blood cells.
Eyam provided O'Brien an ideal opportunity to test this theory. Specifically, Eyam was an isolated population known to have survived a plague epidemic. Everyone in the town would have been exposed to the bacterium, so it's likely that any life-saving genetic trait would have been possessed by each of these survivors. "Like a Xerox machine," says O'Brien, "their gene frequencies have been replicated for several generations without a lot of infusion from outside," thus providing a viable pool of survivor-descendents who would have inherited such a trait.
Knowing who died and who lived through the early years of the plague is somewhat problematic. Deaths among the general English population were not recorded in the 14th Century -- the height of the Plague -- and most communities did not begin recording parish registers until around 1538. Fortunately, Eyam began keeping a parish register in 1630. Thus historian John Clifford began by examining the register, noting everyone who was alive in 1665, the year the plague came to Eyam. He searched for evidence of life through the year 1725 -- marriages, baptisms, burials that took place years after the plague had left the village. Deleting the names of those lost during the plague period, he was
able to determine who the survivors were.
DNA samples could only be collected from direct descendents of the plague survivors. DNA is the principal component of chromosomes, which carry the genes that transmit hereditary characteristics. We inherit our DNA from our parents, thus Eyam resident Joan Plant, for instance, may have inherited the delta 32 mutation from one of her ancient relatives. Plant can trace her mother's lineage back ten generations to the Blackwell siblings, Francis and Margaret, who both lived through the plague to the turn of the 18th century. The next step was to harvest a DNA sample from Joan and the other descendants. DNA is found in the nuclei of cells. The amount is constant in all typical cells, regardless of the size or function of that cell. One of the easiest methods of obtaining a DNA tissue sample is to take a cheek, or buccal, swab.
Scientists studying HIV first learned about the gateway-blocking capacity of the CCR5 mutation in 1996. Several drug companies, then, quickly began exploring the possibility of developing pharmaceuticals that would mimic delta 32 by binding to CCR5 and blocking the attachment of HIV. Previous methods of treatment interfered with HIV's ability to replicate after the virus has already entered a cell. This new class of HIV treatment, called early-inhibitor -- or fusion-inhibitor -- drugs seek to prevent the virus from ever attaching at all. These pharmaceuticals are still in relatively early stages of development, but certainly stand as a hopeful new method of approaching HIV treatment.
Crohn's blood, resisting infection. After three weeks of testing at University College in London, delta 32 had been found in 14% of the samples. This is a genetically significant percentage, yet what, really, did it mean? Could the villagers have inherited delta 32 from elsewhere, residents who had moved to the community in the 350 years since the plague? Was this really a higher percentage than anywhere else? To find out, O'Brien assembled an international team of scientists to test for the presence of delta 32 around the world. "Native Africans did not have delta 32 at all," O'Brien says, "and when we looked at East Asians and Indians, they were also flat zero." In fact, the levels of delta 32 found in Eyam were only matched in regions of Europe that had been affected by the plague and in America, which was, for the most part, settled by European plague survivors and their
descendents.
Meanwhile, recent work with another disease strikingly similar to the plague, AIDS, suggests O'Brien was on the right track. HIV, the virus that causes AIDS, tricks the immune system in a similar manner as the plague bacterium, targeting and taking over white blood cells. Virologist Dr. Bill Paxton at the Aaron Diamond AIDS Research Center in New York City noticed, "the center had no study of people who were exposed to HIV but who had remained negative." He began testing the blood of high-risk, HIV-negative individuals like Steve Crohn, exposing their blood to three thousand times the amount of HIV normally needed to infect a cell. Steve's blood never became infected. "We thought maybe we had infected the culture with bacteria or whatever," says Paxton. "So we went back to Steve. But it was the same result. We went back again and again. Same result." Paxton began studying Crohn's DNA, and concluded there was some sort of blocking mechanism preventing the virus from binding to his cells. Further research showed that that mechanism was delta 32.
Eyam provided O'Brien an ideal opportunity to test this theory. Specifically, Eyam was an isolated population known to have survived a plague epidemic. Everyone in the town would have been exposed to the bacterium, so it's likely that any life-saving genetic trait would have been possessed by each of these survivors. "Like a Xerox machine," says O'Brien, "their gene frequencies have been replicated for several generations without a lot of infusion from outside," thus providing a viable pool of survivor-descendents who would have inherited such a trait.
Knowing who died and who lived through the early years of the plague is somewhat problematic. Deaths among the general English population were not recorded in the 14th Century -- the height of the Plague -- and most communities did not begin recording parish registers until around 1538. Fortunately, Eyam began keeping a parish register in 1630. Thus historian John Clifford began by examining the register, noting everyone who was alive in 1665, the year the plague came to Eyam. He searched for evidence of life through the year 1725 -- marriages, baptisms, burials that took place years after the plague had left the village. Deleting the names of those lost during the plague period, he was
able to determine who the survivors were.
DNA samples could only be collected from direct descendents of the plague survivors. DNA is the principal component of chromosomes, which carry the genes that transmit hereditary characteristics. We inherit our DNA from our parents, thus Eyam resident Joan Plant, for instance, may have inherited the delta 32 mutation from one of her ancient relatives. Plant can trace her mother's lineage back ten generations to the Blackwell siblings, Francis and Margaret, who both lived through the plague to the turn of the 18th century. The next step was to harvest a DNA sample from Joan and the other descendants. DNA is found in the nuclei of cells. The amount is constant in all typical cells, regardless of the size or function of that cell. One of the easiest methods of obtaining a DNA tissue sample is to take a cheek, or buccal, swab.
Scientists studying HIV first learned about the gateway-blocking capacity of the CCR5 mutation in 1996. Several drug companies, then, quickly began exploring the possibility of developing pharmaceuticals that would mimic delta 32 by binding to CCR5 and blocking the attachment of HIV. Previous methods of treatment interfered with HIV's ability to replicate after the virus has already entered a cell. This new class of HIV treatment, called early-inhibitor -- or fusion-inhibitor -- drugs seek to prevent the virus from ever attaching at all. These pharmaceuticals are still in relatively early stages of development, but certainly stand as a hopeful new method of approaching HIV treatment.
Crohn's blood, resisting infection. After three weeks of testing at University College in London, delta 32 had been found in 14% of the samples. This is a genetically significant percentage, yet what, really, did it mean? Could the villagers have inherited delta 32 from elsewhere, residents who had moved to the community in the 350 years since the plague? Was this really a higher percentage than anywhere else? To find out, O'Brien assembled an international team of scientists to test for the presence of delta 32 around the world. "Native Africans did not have delta 32 at all," O'Brien says, "and when we looked at East Asians and Indians, they were also flat zero." In fact, the levels of delta 32 found in Eyam were only matched in regions of Europe that had been affected by the plague and in America, which was, for the most part, settled by European plague survivors and their
descendents.
Meanwhile, recent work with another disease strikingly similar to the plague, AIDS, suggests O'Brien was on the right track. HIV, the virus that causes AIDS, tricks the immune system in a similar manner as the plague bacterium, targeting and taking over white blood cells. Virologist Dr. Bill Paxton at the Aaron Diamond AIDS Research Center in New York City noticed, "the center had no study of people who were exposed to HIV but who had remained negative." He began testing the blood of high-risk, HIV-negative individuals like Steve Crohn, exposing their blood to three thousand times the amount of HIV normally needed to infect a cell. Steve's blood never became infected. "We thought maybe we had infected the culture with bacteria or whatever," says Paxton. "So we went back to Steve. But it was the same result. We went back again and again. Same result." Paxton began studying Crohn's DNA, and concluded there was some sort of blocking mechanism preventing the virus from binding to his cells. Further research showed that that mechanism was delta 32.
Black Death and AIDS are global pandemics that have captured the popular imagination, both attracting extravagant hypotheses to account for their origins and geographical distributions. Medical scientists have recently attempted to connect these two great pandemics. Some argue that the Black Death of 1346-52 was responsible for a genetic shift that conferred a degree of resistance to HIV 1 infection, that this shift was almost unique to European descendents, and that it mirrors the intensity of Black Death mortality within Europe. Such a hypothesis is not supported by the historical evidence: the Black Death did not strike Europe alone but spread from the east, devastating regions such as China, North Africa, and the Middle East as much or even more than Europe. Further, in Europe its levels of mortality do not correspond with the geographic distribution of the proportion of descendents with this CCR5 gene. If anything, the gradient of Black Death mortality sloped in the opposite direction from that of present-day genotypes: the heaviest casualties were in the Mediterranean, the very regions whose descendents account for the lowest incidences of the HIV-1 resistant allele. We argue that closer collaboration between historians and scientists is needed to understand the selective pressures on genetic mutation, and the possible triggers for changes in genetic spatial frequencies over the past millennia. This requires care and respect for each other's methods of evaluating data.
HIV Resistant
The CCR5 chemokine receptor is exploited by HIV-1 to gain entry into CD4+ T cells. A deletion mutation (Delta32) confers resistance against HIV by obliterating the expression of the receptor on the cell surface. Intriguingly, this allele is young in evolutionary time, yet it has reached relatively high frequencies in Europe. These properties indicate that the mutation has been under intense positive selection. HIV-1 has not exerted selection for long enough on the human population to drive the CCR5-Delta32 allele to current frequencies, fueling debate regarding the selective pressure responsible for rise of the allele. The allele exists at appreciable frequencies only in Europe, and within Europe, the frequency is higher in the north. Here we review the population genetics of the CCR5 locus, the debate over the historical selective pressure acting on CCR5-Delta32, the inferences that can potentially be drawn from the geographic distribution of CCR5-Delta32 and the role that other genetic polymorphisms play in conferring resistance against HIV. We also discuss parallel evolution that has occurred at the CCR5 locus of other primate species. Finally, we highlight the promise that therapies based on interfering with the CCR5 receptor could have in the treatment of HIV.
CCR5
C-C chemokine receptor type 5, also known as CCR5 or CD195, is a proteinon the surface of whiteblood cells that is involved in the immune system as it acts as a receptor for chemokines. This is the process by which T cells are attracted to specific tissue and organ targets. Many forms of HIV, the virus that causes AIDS, initially use CCR5 to enter and infect host cells. A few individuals carry a mutation known as CCR5-Δ32 in the CCR5 gene, protecting them against these strains of HIV. In humans, the CCR5 gene that encodes the CCR5 protein is located on the short (p) arm at position 21 on chromosome 3. Certain populations have inherited the Delta 32 mutation resulting in the genetic deletion of a portion of the CCR5 gene. Homozygous carriers of this mutation are resistant to M-tropic strains of HIV-1 infection.

